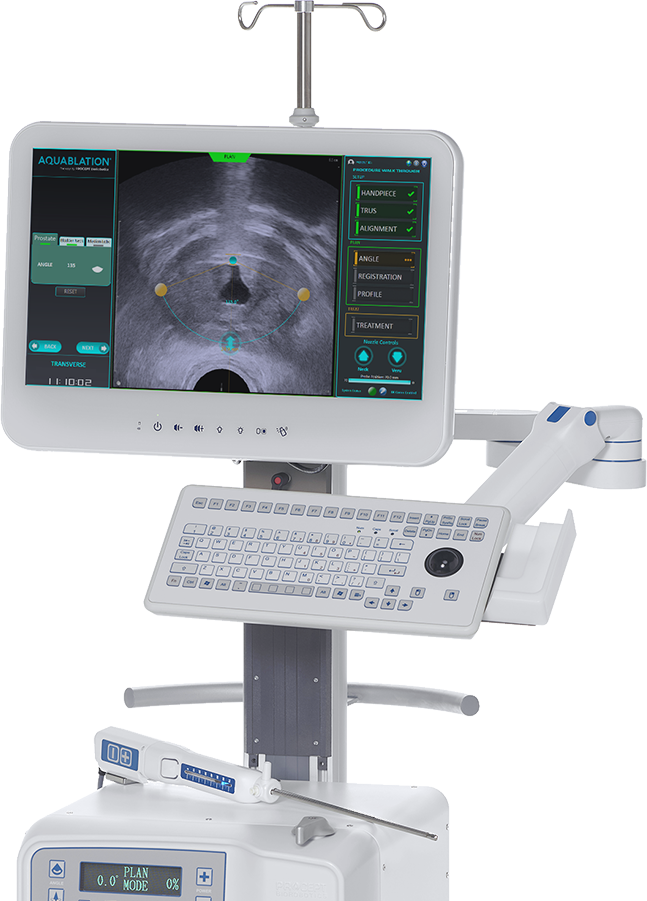
AquaBeam Autonomous Surgical Robots for BPH Treatment
The first and only image-guided, heat-free robotic therapy for the treatment of lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH).
Next-Generation Robotic Technology
The AquaBeam Robotic system is an advanced, image-guided, surgical robotic system that combines real-time, multidimensional imaging, personalized treatment planning, automated robotics and heat-free waterjet ablation for precise and rapid removal of prostate tissue.
The AquaBeam Robotic System delivers Aquablation therapy, a minimally invasive BPH surgery that addresses the compromise between safety and efficacy of alternative surgical interventions.
The Aquablation Procedure
Aquablation therapy is performed in the operating room using the AquaBeam Robotic System, while the patient is either under spinal or general anesthesia. Real-time, multidimensional imaging facilitates personalized treatment planning, automated robotic technology allows for predictability and reproducibility of the procedure, and heat-free water jet technology enables precise removal of obstructive prostate tissue.
Real-Time, Multidimensional Imaging
Aquablation therapy is unique in that it combines cystoscopic visualization, ultrasound imaging and advanced planning software to provide the surgeon with a multidimensional view of the treatment area. This enables personalized treatment planning for the patient’s unique anatomy, improved decisionmaking and real-time monitoring during the procedure.
Real-Time, Multidimensional Imaging
Aquablation therapy is unique in that it combines cystoscopic visualization, ultrasound imaging and advanced planning software to provide the surgeon with a multidimensional view of the treatment area. This enables personalized treatment planning for the patient’s unique anatomy, improved decisionmaking and real-time monitoring during the procedure.
Personalized Treatment Planning
With a multidimensional view of the prostate, the surgeon creates a map of the treatment area, tailored to each patient’s anatomy, that specifies which areas of the prostate to remove while preserving the anatomy that controls erectile function, ejaculatory function, and continence.
Robotic Tissue Resection with a Heat-Free Waterjet
Once the treatment map is complete, prostate tissue is precisely removed using a robotically controlled, heat-free waterjet. The robotic and waterjet technologies enable targeted and controlled tissue removal with rapid resection times that are highly consistent across all prostate sizes and shapes, and surgeon experience levels.
In clinical studies, Aquablation therapy delivers outcomes that are effective, safe and durable across all prostate sizes and shapes.
Robotic Tissue Resection with a Heat-Free Waterjet
Once the treatment map is complete, prostate tissue is precisely removed using a robotically controlled, heat-free waterjet. The robotic and waterjet technologies enable targeted and controlled tissue removal with rapid resection times that are highly consistent across all prostate sizes and shapes, and surgeon experience levels.
In clinical studies, Aquablation therapy delivers outcomes that are effective, safe and durable across all prostate sizes and shapes.